Equitable Access to Therapies on a Global Scale
Executive Summary
Access to treatments is a fundamental right of all people living with ALS/MND yet there is an urgent need for Equitable Access. On a global scale, it takes too long for new treatments to slowly make their way through the traditional pathways from clinical research to market access and many countries lack the infrastructure to support clinical trials, or resources to provide the funding and delivery of therapies. There is little to no collaborative engagement between regulators in each country. There is unmet urgency from the ALS/MND community.
The availability of clinical trials differs from country to country and there are several barriers to accessing clinical trials that disproportionately affect certain populations, including people who reside in developing countries and remote areas with limited access health centres.
Additionally, Special Access Programs are often available only in high-income countries, resulting in limited access to treatments for patients in low and middle-income countries. And, within countries that provide public drug programs, people living with ALS/MND must often wait years through a series of drawn-out, confusing, red-tape-filled delays caused by sequential reviews and hand-offs before decisions on access are made.
The current lack of global regulatory collaboration creates significant disparities in access to treatment and results in inequitable health outcomes for people living with ALS/MND. Furthermore, it slows down the development and approval of new therapies, leading to limited treatment options for patients.
Addressing these disparities requires efforts to ensure that people living with ALS/MND have access to safe and effective treatments, regardless of where they live and at minimal cost. This requires collaboration among regulators, healthcare providers, industry and policymakers to develop strategies to improve access to treatments and ensure that people living with ALS/MND receive the best possible care.
Given the recent scientific and clinical progress in the ALS/MND space, where new potentially efficacious therapies are emerging through successful clinical trials, the International ALS/MND Alliance supports the development of a cooperative global framework to create an environment for increased clinical trial availability and evaluate the risks and benefits of new therapies, especially given the global nature of drug development and clinical trial conduct.
Rationale for Support
Access to the highest quality treatment available is a fundamental right for people living with ALS/MND. The Fundamental Rights of people with ALS/MND does not stand in isolation of other overarching imperatives including the United Nations Sustainable Development Goals. The 2030 Agenda for Sustainable Development, adopted by all United Nations Member States in 2015, provides a shared blueprint for peace and prosperity for people and the planet, now and into the future. At its heart are the 17 Sustainable Development Goals (SDGs), which are an urgent call for action by all countries – developed and developing – in a global partnership. They recognize that ending poverty and other deprivations must go hand-in-hand with strategies that improve health and education, reduce inequality, and spur economic growth – all while tackling climate change and working to preserve our oceans and forests. The Alliance’s Fundamental Rights are further supported by the World Medical Association (WMA) Declaration of Lisbon on the Rights of the Patient which states that “Every person is entitled without discrimination to appropriate medical care.” (See Appendix 2) In addition, this fundamental right is embedded in UNESCO’s Universal Declaration on Bioethics and Human Rights. In particular, Article 15 which states:
“Sharing of benefits:
Benefits resulting from any scientific research and its applications should be shared with society as a whole and within the international community, in particular with developing countries. In giving effect to this principle, benefits may take any of the following forms:
- special and sustainable assistance to, and acknowledgement of, the persons and groups that have taken part in the research;
- access to quality health care;
- provision of new diagnostic and therapeutic modalities or products stemming from research;
- support for health services;
- access to scientific and technological knowledge;
- capacity-building facilities for research purposes;
- other forms of benefit consistent with the principles set out in this Declaration.”
Pathways to Access
Not all countries may be able to meet the basic needs of people living with ALS/MND or there may be limited availability. Within the current access environment, each country or geography has its own processes for investing in, approving, and making treatments available. The following graphic illustrates the Pathway to Access continuum:
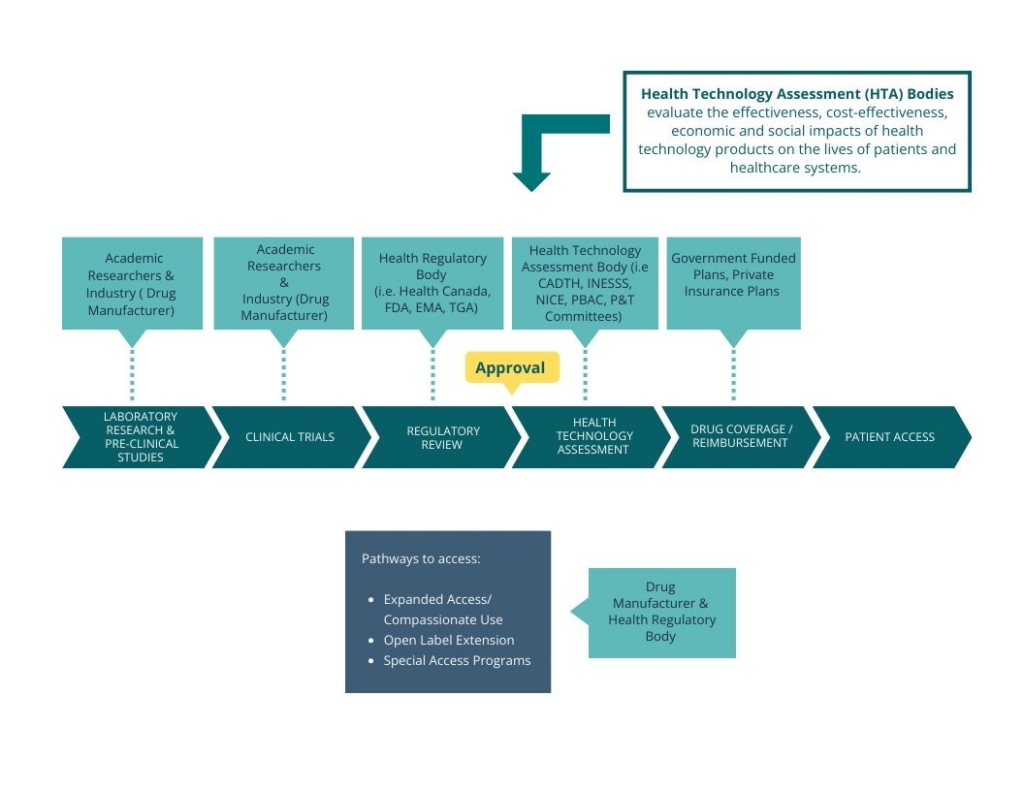
- Clinical Trials: Clinical trials are an opportunity for people to try new therapies while still within the research phase. Participation in clinical trials is crucial for people living with ALS/MND as it can provide them with access to cutting-edge therapies that could have therapeutic benefits, the opportunity to contribute to future treatment development and access to specialized medical care.
- Expanded Access Programs/Compassionate Use: Is a potential pathway for a patient to gain access to an investigational treatment outside of a clinical trial.
- Open Label Extension Studies: Open label extension studies are an opportunity for people living with ALS/MND to continue to have access to the experimental therapy once their participation in a clinical trial is complete. The programs/studies eliminate any gaps in treatment and enable the person to continue treatment under clinical monitoring.
- Special Access Programs (SAP): Special access programs are designed to provide people access to therapies that have not yet been approved in their country or are not yet available through a reimbursed pathway. Manufacturers must agree to provide access through this program, often at no cost.
- Regulatory Approval: Regulatory approval is the process by which the health authority reviews a drug for safety, efficacy, and quality to determine whether it can be approved for sale in the country. A manufacturer must apply directly to the health authority for drug approval.
- Drug Reimbursement: In some countries, medical care – including the cost of drugs – is covered by public insurance programs. Once a drug has been approved for sale, there may be an additional process that evaluates the clinical and economic evidence (Health Technology Assessment – HTA) of the drug to determine whether state-sponsored (or public) drug programs will cover the cost of the drug.
Private insurance companies also undertake their own review of a drug in order to decide whether they will reimburse the cost through their insurance program. These decisions are specific to the company and again within the individual drug plans offered.
None of these pathways create an environment where there is equitable access to therapies for anyone who can benefit – nor do they reflect the urgency of living with ALS/MND.
Impact and Influence
Amyotrophic lateral sclerosis (ALS) or motor neuron disease (MND) is a relentless, fatal motor neuron disease that involves the brain and spinal cord, the body’s muscles, and the motor neurons that send signals between the two. The disease moves with startling swiftness, causing progressive paralysis. Over a two-to-five-year period, on average, someone with ALS/MND will lose the ability to walk, talk, eat, move, swallow – and breathe.
Receiving an ALS/MND diagnosis is devastating, and the disease’s physical, emotional, and financial impacts on a person and their family are immense. Yet, the unique and complex care needs of people living with ALS/MND continue to go unmet regardless of where they live – especially as it relates to opportunities for timely access to potentially beneficial therapies.
We recognize many pathways as a drug moves through to market availability. As such, we want to ensure that people living with ALS/MND can leverage as many pathways as possible. There are points all along the Pathway to Access Continuum that Patient Advocacy Groups and ALS/MND Organizations can have effective impact.
- Clinical Trials: There are several barriers to accessing clinical trials that are inequitable and disproportionately affect certain populations, including individuals who reside in developing countries and remote areas with limited access to trials. Local health authorities must provide support for multidisciplinary clinics within their region capable of supporting clinical trials. Clinical trial sponsors must also work with ALS/MND clinicians on a global scale to ensure clinical trials sites are established in as many countries as possible. Access to clinical trials is a vital and necessary step in ensuring the fundamental right of access to the highest quality treatment. The Alliance’s research strategy will help expand access to clinical trial sites particularly in the Global South.
The PALS & CALS Advisory Council of the International Alliance would emphasize that clinical trials should not economically disadvantage participants and that wherever possible the cost of each trial must include the cost paid by the patients to participate. Parking, travelling, hotel and other cost should be included when in-person is necessary, and as much telehealth as possible should be embedded to minimize distance from the lab as an obstacle for participating. Infrastructure should be local and equipment and materials available to minimize disruption. - Open Label Extension Studies: The Alliance believes that open label extension studies must be incorporated into all clinical trial design and protocols to ensure people living with ALS/MND can continue to access experimental therapies once their participation in a clinical trial has ended. Open Label Extension studies are critical to ensuring there is not disruption to the course of treatment and are vital to collecting real-world evidence to help support clinical care on a global scale.
- Expanded Access Programs/Compassionate Use: Manufacturers should take into account the opportunity for the global ALS/MND population to access therapies prior to market-access availability.
- Special Access Programs (SAP): SAPs are often available only in high-income countries, resulting in limited access to treatments for patients in low and middle-income countries, creating significant disparities in access to treatment and results in inequitable health outcomes for people living with ALS/MND. Manufacturers must consider utilizing special access programs, especially in low and middle-income countries or where access is delayed due to lengthy reimbursement processes.
- Regulatory Approval: Currently, each country or geography has its own regulatory process for approving ALS/MND therapies and there is little collaborative engagement with other parts of the world. However, as demonstrated through other disease areas, such as oncology, global models for regulatory approval can successfully bring effective therapies to people as early as possible, especially in countries where there may otherwise have been delays with regulatory submission and review. Given the recent scientific and clinical progress in the ALS/MND space, where new potentially efficacious therapies are emerging through successful clinical trials, the time is right for the Alliance to take on the opportunity and the responsibility for advancing an alternative, more harmonized global approach.
The International ALS/MND Alliance seeks to encourage all international regulatory agencies to develop a framework for global alignment in evaluating potential new therapies and will work to develop a cooperative, harmonized, pathway to approval that increases effectiveness and expediency for treatments of ALS/MND. - Drug Reimbursement: Streamlined and swift drug reimbursement must be included as part of any global strategy to improve access to innovative therapies. Once an ALS/MND therapy is approved, it must be reviewed for public reimbursement, and covered through a single condensed timeframe applicable to all jurisdictions.
A harmonized global approach that allows for timely access to therapies will uphold the rights of those individuals with ALS/MND to have equitable access new treatments and clinical trials, in both developing and developed countries. It will benefit not only the ALS/MND community, but all stakeholders involved in therapy development. Furthermore, with greater access to therapies and clinical trials, disease progression will be slowed, and death could be delayed; independence and quality of life will be retained; and perhaps with this extension of life, new therapies will become available that will cure the disease.
International Alliance of ALS/MND Associations
October 2023
The original language of communication is English and any translation cannot be guaranteed for accuracy of messaging.
APPENDICES
Appendix 1: Fundamental Rights of People with ALS/MND

Appendix 2: WMA Declaration of Lisbon on the Rights of the Patient
https://www.wma.net/policies-post/wma-declaration-of-lisbon-on-the-rights-of-the-patient/
Adopted by the 34th World Medical Assembly, Lisbon, Portugal, September/October 1981 and amended by the 47th WMA General Assembly, Bali, Indonesia, September 1995 and editorially revised by the 171st WMA Council Session, Santiago, Chile, October 2005 and reaffirmed by the 200th WMA Council Session, Oslo, Norway, April 2015
PREAMBLE
The relationship between physicians, their patients and broader society has undergone significant changes in recent times. While a physician should always act according to his/her conscience, and always in the best interests of the patient, equal effort must be made to guarantee patient autonomy and justice. The following Declaration represents some of the principal rights of the patient that the medical profession endorses and promotes. Physicians and other persons or bodies involved in the provision of health care have a joint responsibility to recognize and uphold these rights. Whenever legislation, government action or any other administration or institution denies patients these rights, physicians should pursue appropriate means to assure or to restore them.
PRINCIPLES
- Right to medical care of good quality
- Every person is entitled without discrimination to appropriate medical care.
- Every patient has the right to be cared for by a physician whom he/she knows to be free to make clinical and ethical judgements without any outside interference.
- The patient shall always be treated in accordance with his/her best interests. The treatment applied shall be in accordance with generally approved medical principles.
- Quality assurance should always be a part of health care. Physicians, in particular, should accept responsibility for being guardians of the quality of medical services.
- In circumstances where a choice must be made between potential patients for a particular treatment that is in limited supply, all such patients are entitled to a fair selection procedure for that treatment. That choice must be based on medical criteria and made without discrimination.
- The patient has the right to continuity of health care. The physician has an obligation to cooperate in the coordination of medically indicated care with other health care providers treating the patient. The physician may not discontinue treatment of a patient as long as further treatment is medically indicated, without giving the patient reasonable assistance and sufficient opportunity to make alternative arrangements for care.
- Right to freedom of choice
- The patient has the right to choose freely and change his/her physician and hospital or health service institution, regardless of whether they are based in the private or public sector.
- The patient has the right to ask for the opinion of another physician at any stage.
- Right to self-determination
- The patient has the right to self-determination, to make free decisions regarding himself/herself. The physician will inform the patient of the consequences of his/her decisions.
- A mentally competent adult patient has the right to give or withhold consent to any diagnostic procedure or therapy. The patient has the right to the information necessary to make his/her decisions. The patient should understand clearly what is the purpose of any test or treatment, what the results would imply, and what would be the implications of withholding consent.
- The patient has the right to refuse to participate in research or the teaching of medicine.
- The unconscious patient
- If the patient is unconscious or otherwise unable to express his/her will, informed consent must be obtained whenever possible, from a legally entitled representative.
- If a legally entitled representative is not available, but a medical intervention is urgently needed, consent of the patient may be presumed, unless it is obvious and beyond any doubt on the basis of the patient’s previous firm expression or conviction that he/she would refuse consent to the intervention in that situation.
- However, physicians should always try to save the life of a patient unconscious due to a suicide attempt.
- The legally incompetent patient
- If a patient is a minor or otherwise legally incompetent, the consent of a legally entitled representative is required in some jurisdictions. Nevertheless the patient must be involved in the decision-making to the fullest extent allowed by his/her capacity.
- If the legally incompetent patient can make rational decisions, his/her decisions must be respected, and he/she has the right to forbid the disclosure of information to his/her legally entitled representative.
- If the patient’s legally entitled representative, or a person authorized by the patient, forbids treatment which is, in the opinion of the physician, in the patient’s best interest, the physician should challenge this decision in the relevant legal or other institution. In case of emergency, the physician will act in the patient’s best interest.
- Procedures against the patient’s will
Diagnostic procedures or treatment against the patient’s will can be carried out only in exceptional cases, if specifically permitted by law and conforming to the principles of medical ethics. - Right to information
- The patient has the right to receive information about himself/herself recorded in any of his/her medical records, and to be fully informed about his/her health status including the medical facts about his/her condition. However, confidential information in the patient’s records about a third party should not be given to the patient without the consent of that third party.
- Exceptionally, information may be withheld from the patient when there is good reason to believe that this information would create a serious hazard to his/her life or health.
- Information should be given in a way appropriate to the patient’s culture and in such a way that the patient can understand.
- The patient has the right not to be informed on his/her explicit request, unless required for the protection of another person’s life.
- The patient has the right to choose who, if anyone, should be informed on his/her behalf.
- Right to confidentiality
- All identifiable information about a patient’s health status, medical condition, diagnosis, prognosis and treatment and all other information of a personal kind must be kept confidential, even after death. Exceptionally, descendants may have a right of access to information that would inform them of their health risks.
- Confidential information can only be disclosed if the patient gives explicit consent or if expressly provided for in the law. Information can be disclosed to other health care providers only on a strictly “need to know” basis unless the patient has given explicit consent.
- All identifiable patient data must be protected. The protection of the data must be appropriate to the manner of its storage. Human substances from which identifiable data can be derived must be likewise protected.
- Right to Health Education
Every person has the right to health education that will assist him/her in making informed choices about personal health and about the available health services. The education should include information about healthy lifestyles and about methods of prevention and early detection of illnesses. The personal responsibility of everybody for his/her own health should be stressed. Physicians have an obligation to participate actively in educational efforts. - Right to dignity
- The patient’s dignity and right to privacy shall be respected at all times in medical care and teaching, as shall his/her culture and values.
- The patient is entitled to relief of his/her suffering according to the current state of knowledge.
- The patient is entitled to humane terminal care and to be provided with all available assistance in making dying as dignified and comfortable as possible.
- Right to religious assistance
The patient has the right to receive or to decline spiritual and moral comfort including the help of a minister of his/her chosen religion.
Appendix 3: Universal Declaration on Bioethics and Human Rights
https://www.unesco.org/en/legal-affairs/universal-declaration-bioethics-and-human-rights?hub=66535
Article 10
Equality, justice and equity
The fundamental equality of all human beings in dignity and rights is to be respected so that they are treated justly and equitably.
Article 15
Sharing of benefits
- Benefits resulting from any scientific research and its applications should be shared with society as a whole and within the international community, in particular with developing countries. In giving effect to this principle, benefits may take any of the following forms:
- special and sustainable assistance to, and acknowledgement of, the persons and groups that have taken part in the research;
- access to quality health care;
- provision of new diagnostic and therapeutic modalities or products stemming from research;
- support for health services;
- access to scientific and technological knowledge;
- capacity-building facilities for research purposes;
- other forms of benefit consistent with the principles set out in this Declaration.
- Benefits should not constitute improper inducements to participate in research.
Article 22
Role of States
- States should take all appropriate measures, whether of a legislative, administrative or other character, to give effect to the principles set out in this Declaration in accordance with international human rights law. Such measures should be supported by action in the spheres of education, training and public information.
- States should encourage the establishment of independent, multidisciplinary and pluralist ethics committees, as set out in Article 19.