Background
In 1993, mutations in the cytosolic copper-zinc superoxide dismutase 1 (SOD1) gene were discovered to be associated with ALS (Ref: Rosen et al., 1993) accounting for roughly 2% of all ALS cases and approximately 15-20% of ALS/MND with familial history. The SOD1 gene contains the instructions needed to produce the SOD1 protein, an abundant enzyme within cells that serves to keep them safe from metabolic waste. Mutations in the SOD1 gene cause an accumulation of defective SOD1 protein in patients’ cells. These defective proteins create a toxic environment and result in motor neuron death (Ref: Bunton-Stasyshyn et al., 2014).
To date both the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) granted extraordinary approval to Qalsody (tofersen) to treat patients affected by ALS associated with a mutation in SOD1 (SOD1-ALS). Qalsody is an antisense oligonucleotide (ASO) directed against SOD1 messenger RNA (mRNA), which encodes the SOD1 protein. By targeting the SOD1 mRNA, this ASO reduces the overall expression of the SOD1 protein, limiting its toxic activity in people with SOD1-ALS. Qalsody is administered through an injection in the spinal fluid (intrathecally) every 28 days (Ref: FDA). More information on trial design and outcomes that determined FDA approval can be found at als-mnd.org (Biogen-tofersen).
The ATLAS Trial
Early intervention has long been considered as likely optimal in ALS/MND, though it has never been clinically tested. The ability to initiate experimental and proven treatments upstream of clinical symptom onset is a milestone that requires a biological indicator (biomarker) of underlying disease processes being triggered.
In recent years, a significant amount of work has yielded the protein called neurofilament light chain (NfL) as a potential blood biomarker to indicate that nervous system damage has occurred. While this is not specific for ALS, when combined with known, disease-causing genetic mutations, it may provide an opportunity to visualize the pre-clinical triggering of ALS/MND processes.
Given tofersen’s efficacy and safety profile, and the hypothesized advantage of early therapeutic intervention in ALS/MND, Biogen started a global pre-symptomatic trial in ~150 individuals who carry selected SOD1 mutations linked to rapid disease progression but have not yet shown any ALS symptoms. This is a Phase 3 randomized, placebo-controlled trial, and results are expected in 2027. The leading hypothesis for this trial is that by reducing production of toxic SOD1 protein in people with SOD1 mutations, the drug will either delay symptoms from appearing or prevent symptoms altogether. Participants are monitored monthly for change in ALS Functional Rating Scale (ALSFRS-R) total score, change from baseline in percent predicted Slow Vital Capacity (SVC), adverse events and plasma and CSF-based biomarkers. Specifically, participants are monitored monthly for any change in plasma-based biomarkers called neurofilament light (NfL), which indicates early stage of disease up to a year prior symptom onset. If in any of the subjects’ treated with placebo in the people who show symptoms within a year. All individuals on placebo will be provided open label tofersen upon an ALS diagnosis, which still results in earlier access to the treatment than any of the individuals affected by SOD1-ALS treated to date (Ref: ClinicalTrials.gov, ID: NCT04856982).
The novel primary measure of evaluation will be the proportion of participants who develop clinical symptoms of ALS/MND within one year of randomization. Given that the participants will have SOD1 mutations associated with rapid progression, if a significant number do not have clinical symptoms after one year, it would suggest that tofersen is able to delay the disease process. Participants will be treated for up to two years as part of the study.
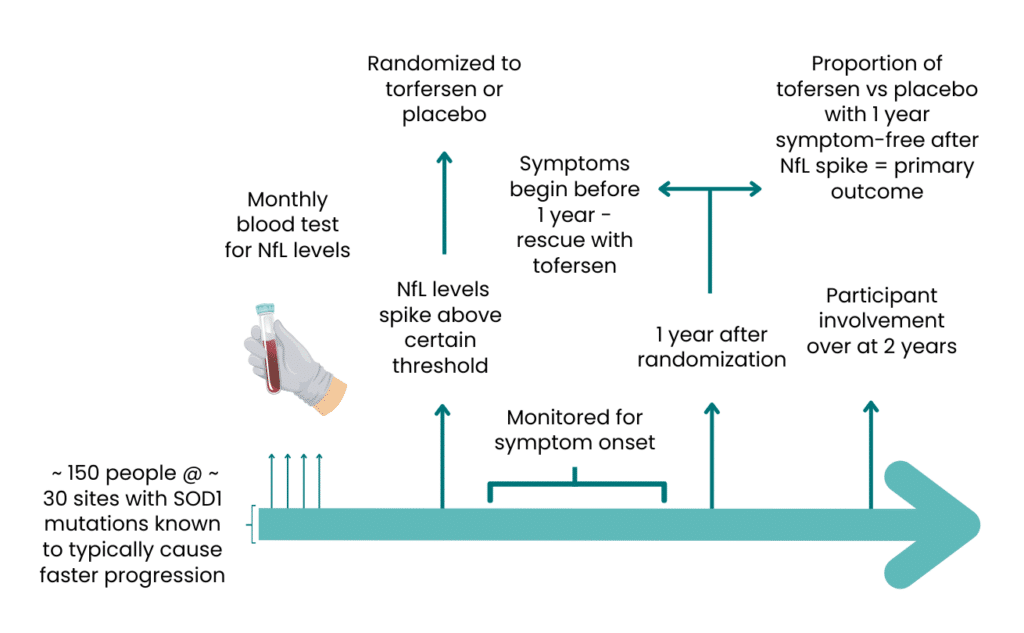
Fig1. ATLAS trial design overview
It is hoped that the ATLAS trial will pave the way for more pre-symptomatic trials in the future. Should therapies become proven as effective for other known genetic mutations, these pre-symptomatic studies may indicate the next logical step and will have learned from ATLAS in the effectiveness of using NfL as a trial initiation biomarker in practice.
For cases where there is no identifiable mutation in a known ALS gene, researchers will need to identify additional biomarker(s) that can differentiate between nervous system damage indicating ALS versus that of many other conditions. As of 2024, there is nothing fitting this criterion that is close to clinical use, but a strong effort is underway in labs around the world.
Other SOD-1 targeting therapies
In addition to the above, three other companies are developing more therapeutics to target SOD1:
- UniQure has developed a one-time intrathecally administered gene therapy (AMT-162), composed by a viral vector (AAVrh10) that expresses a micro ribonucleic acid (miRNA) designed to decrease the expression of SOD1. As of 2024, the FDA has cleared the investigational new drug (IND) application for AMT-162 and granted Orphan Drug and Fast Track designations. UniQure expects to initiate patient dosing in the first quarter of 2024 for a Phase 1/2, multi-center, three-part study (Ref: UniQure Website and ClinicalTrials.gov ID: NCT06100276).
- In 2022 Alnylam Pharmaceuticals published a novel small interfering RNA (siRNA) technology to enhance delivery to the central nervous system (CNS). The publication showed uptake of SOD1 targeting siRNA in the CNS and subsequent lower expression of SOD1 by 75% in a mouse model system (Ref: Brown at al., 2022, Alnylam Pharmaceutical Website). Alnylam is currently collaborating with Regeneron to start a proof-of-concept study in SOD-1 ALS in 2024 (Ref: Regeneron JPM 2024 presentation)
- AviadoBio has recently acquired rights for Neurgian Technologie’s novel approach to deliver gene therapies directly to the spinal cord through a ‘subpial’ injection. This technique allows to get through the membrane that surrounds the spinal cord, resulting in the need for lower doses of compound though a minimally invasive procedure (Ref: Miyanohara et al., 2016, ALS News Today). AviadoBio is aiming to use this method to deliver a short hairpin RNA (shRNA) directed against SOD1 to repress gene expression. Neurgian Technologies showed that in rodents one subpial injection with a virally delivered shRNA resulted in the prevention of the disease in SOD1 carriers and improvement of the disease in symptomatic mice. In pigs and non-human primates, one injection produces homogeneous delivery in the whole spinal cord (Ref: Bravo-Hernandez et al., 2019).
Summary
Therapies that lower the total amount of SOD1 are believed to be an effective strategy against SOD1-ALS. It is expected that we will see an optimization of these therapies, such as earlier intervention or improvement of drug delivery to the CNS. There is still much to learn about the long-term implication of lowering of SOD1 levels and to what extent it can safely be lowered. Furthermore, strategies to lower only the abnormal SOD1 are also a possibility, which would be more specific to SOD1 toxicity in ALS.
It is important to note that there are many areas of the world where tofersen is not a therapeutic option due to different reasons. We hope that as more SOD1 targeting therapies are developed, this will give individuals affected by SOD1-ALS an option to receive the new SOD-1 targeting therapeutics through clinical trials. The Alliance is actively working to create clinical trial networks in geographic regions where they do not already exist to facilitate the diffusion of promising therapies without geographic restrictions.
To date, therapies that lower the total amount of SOD1 protein have only been tested in individuals affected by SOD1-ALS or preclinical models of SOD1-ALS. Therefore, none of the therapies mentioned here are currently indicated for people with ALS who do not carry SOD1 genetic variants. Tofersen is the only FDA and EMA approved drug for the treatment of people affected by ALS associated with a genetic variant in SOD1.
Sources
Rosen et al., 1993 – https://pubmed.ncbi.nlm.nih.gov/8446170/
Bunton-Stasyshyn et al., 2014 – https://doi.org/10.1177/1073858414561795
ClinicalTrial.gov (ATLAS) – https://clinicaltrials.gov/study/NCT04856982#participation-criteria
Businesswire (press release) – https://www.businesswire.com/news/home/20230412005207/en/Arrowhead-Pharmaceuticals-to-Host-RD-Day-on-Pipeline-of-RNAi-Therapeutics
Uniqure website – https://www.uniqure.com/programs-pipeline/als-sod1
ClinicalTrial.gov ID: NCT06100276 – https://clinicaltrials.gov/study/NCT06100276?cond=ALS&term=SOD1&rank=6
Brown at al., 2022 – https://www.nature.com/articles/s41587-022-01334-x
Alnylam Pharmaceuticals Website (press release) – https://investors.alnylam.com/press-release?id=26761
Regeneron JPM 2024 presentation – https://investor.regeneron.com/static-files/7dfdabe2-05d1-4145-b5c5-342cec0ce9c4
Miyanohara et al. 2016 – https://www.ncbi.nlm.nih.gov/pubmed/27462649
ALS News Today – https://alsnewstoday.com/news/aviadobio-acquires-delivery-technology-als-gene-therapies/
Bravo-Hernandez et al., 2019 – https://www.nature.com/articles/s41591-019-0674-1